Insights
What is FSHD?
Facioscapulohumeral muscular dystrophy, or FSHD, is a genetic disorder that leads to the relentless weakening of skeletal muscles. Typically beginning in early teenage years with the loss of muscles in the face (facio), shoulders (scapula), upper arms (humerus), legs or core, FSHD can spread to any muscle. Around 20 percent will need a wheelchair by age 50. Over 70 percent experience debilitating pain and fatigue. FSHD is estimated to affect nearly one million people worldwide. For more information about FSHD, go to fshdsociety.org/what-is-fshd .
About Us
Our mission is to accelerate the development of effective FSHD therapeutics and get treatments to people faster.
Who We Are
Global FSHD Innovation Hub is a wholly-owned subsidiary of the FSHD Society’s 501(c)3 founded in 1991. The Hub is a commercial Limited-Liability Company (LLC) that contracts with biopharma companies to accelerate their product development lifecycle. It operates as a partnership entity to streamline client engagement and contracting processes.
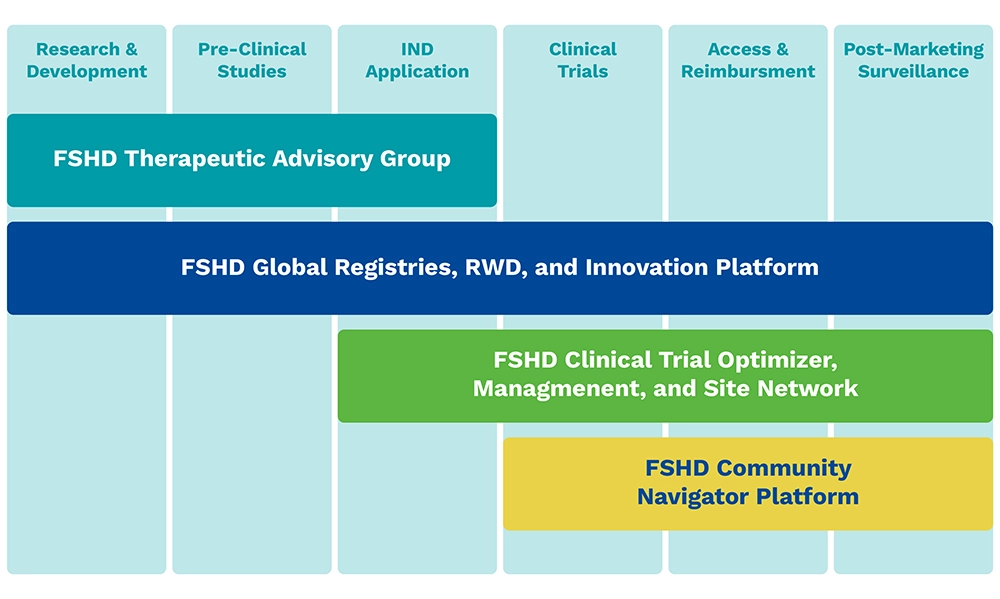
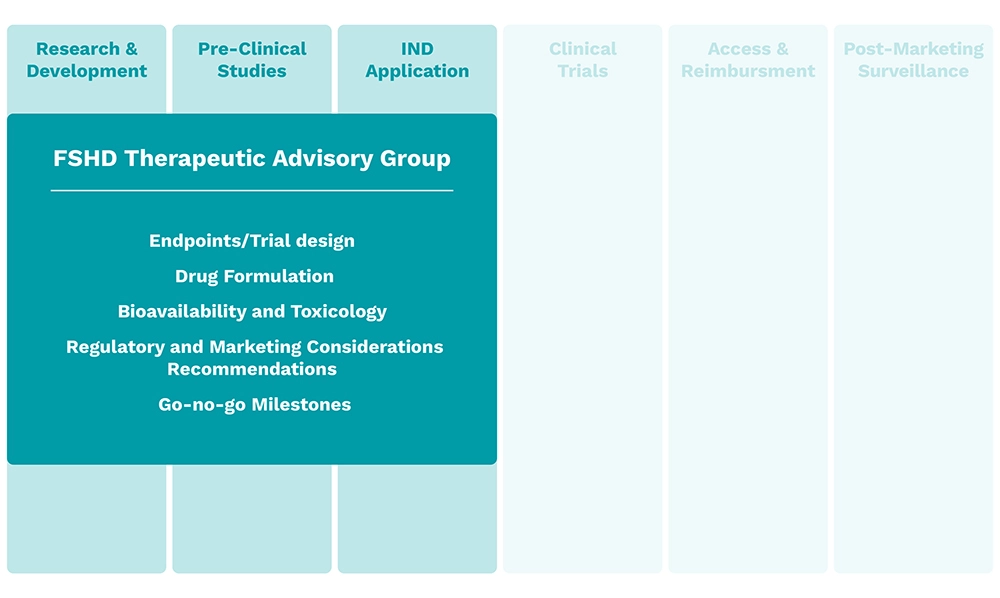
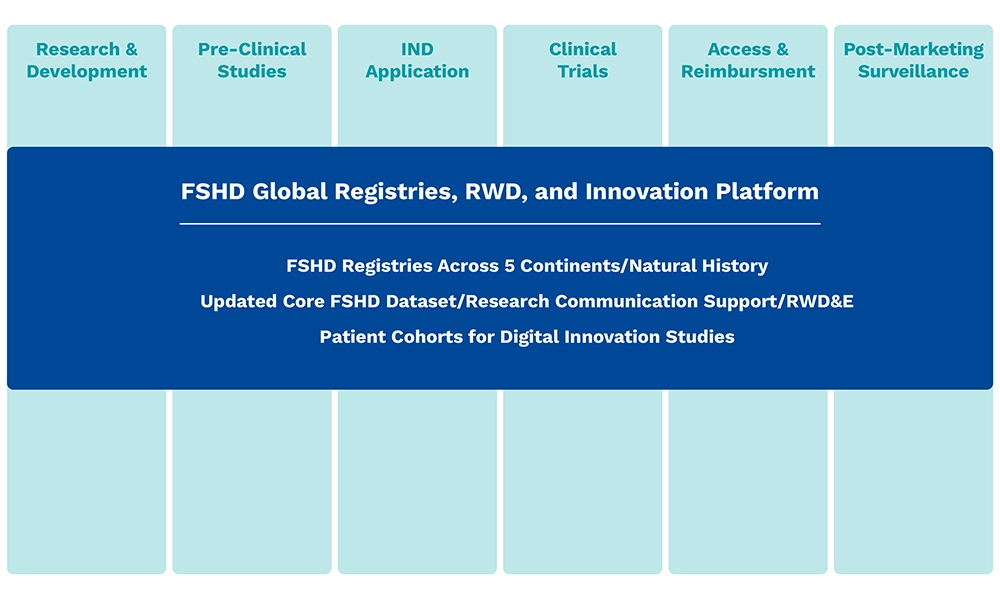
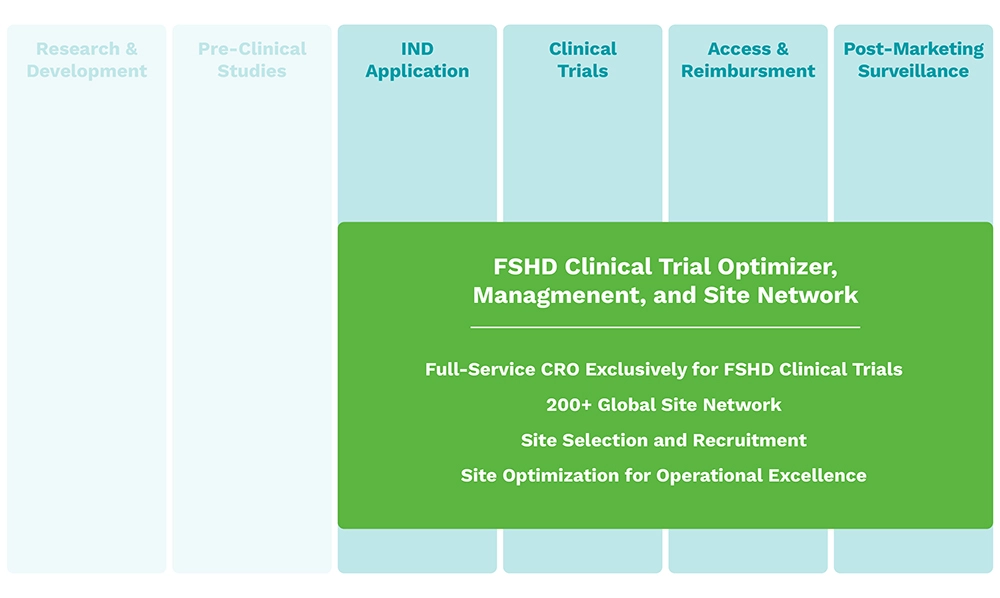
Hub Delivery Partners
Hub Leadership
The Hub leadership team have extensive experience in neuromuscular diseases, clinical services, research, and patient engagement — ensuring a robust foundation for mission.

Amanda Rickard
MANAGING DIRECTOR
Amanda is the Managing Director of the Hub. She brings over a 15 years of experience supporting novel therapeutics development for patients with rare diseases including FSHD, cancers, and autoimmune diseases. Her background includes scientific, business development, and alliance management roles across academic, patient advocacy, biotech, and large pharmaceutical organizations. She leads the Hub team and its engagements with biopharma and technology partners.

Ken Kahtava
CONSULTANT
Ken is a consultant with the Hub. A serial technology and business entrepreneur, Ken is also a veteran leader in the nonprofit research industry with more than twenty years’ experience in establishing public-private partnerships in multiple rare disease communities to accelerate research and access.

Lawrence Korngut, MD MSc FRCPC
CHIEF MEDICAL OFFICER
Lawrence is a clinician investigator at the University of Calgary where he conducts research in registry best practices at the Korngut Registry Science Lab. He led the development of the Canadian Neuromuscular Disease Registry which includes 48 participating clinics across Canada which has successfully recruited over 6000 patients across four disease sub registries. Lawrence is a recognized key opinion leader in FSHD and co-Founder of Lumiio.

Lauren Morgenroth
DIRECTOR OF CLINICAL SERVICES
Lauren led the development of TRiNDS starting in 2016. Prior to that in 2004, she joined the Cooperative International Neuromuscular Research Group (CINRG), an academic clinical trial consortium for which TRiNDS is also the Coordinating Center. Lauren is a genetic counselor and worked in both pediatric and adult muscular dystrophy associations clinics. Lauren is CEO of TRiNDS.

Victoria Hodgkinson, PhD
CHIEF SCIENTIFIC OFFICER
Victoria is CSO at Lumiio and the FSHD Global Innovation Hub. A recognized global expert in registries following her work on large-scale international projects such as the Global SMA Registry and the Global Acromegaly Registry. Her experience in registries includes dataset derivation, protocol and ethics, privacy legislation, and platform design. Victoria also has extensive and varied experience in data analysis and publishing findings utilizing real-world registry data. Victoria co-leads the implementation of platform user requirements in addition to leading ongoing registry projects for clients.

Blaine Penny
DIRECTOR, INNOVATION AND RWD/E
Blaine is CEO of Lumiio and leads project management for the FSHD Global Innovation Hub. He has over 20 years of experience in the health, technology, and engineering sectors leading and managing large teams and portfolios, capital investment projects, product management, M&A, and go-to-market strategies. Fifteen of those years were in Executive roles and included Co-founder and CEO of MitoCanada, CEO of the start-up engineering firm Integrated Sustainability, and Vice President of the global technology firm IHS Markit.
Hub Board of Directors

Mark Stone
CEO of FSHD Society and has served as an executive leader of research-focused patient advocacy nonprofit organizations in multiple rare diseases since 2004. Mark has launched drug discovery initiatives anchored by clinical trial networks globally to expedite potential treatments across multiple diseases communities.

Mel Hayes
Mel Hayes has over three decades of executive leadership experience in building and leading successful commercial and development organizations at major pharmaceutical and biotech companies including Bioverativ/Sanofi, and Fulcrum Therapeutics. With a strong record in rare disease launches and corporate strategy, he brings valuable expertise in advocacy and commercialization that supports the Hub's ability to translate research into real-world impact.

Hans Van Bylen
Hans Van Bylen is a global business leader with more than three decades of executive experience in the consumer goods and chemical industries. A former CEO of Henkel, he led major acquisitions and strategic growth initiatives that expanded the company's international reach. He is currently Chairman of Ontex and Etex and a board member of Lanxess and AkzoNobel. His leadership and global perspective will strengthen the Hub's mission to advance FSHD drug development.

Neil Camarta
Neil Camarta is a chemical engineer and member of the Canadian Academy of Engineering who has held senior leadership positions across the oil and gas industry. He co-founded Western Hydrogen and Enlighten Innovations, two cleantech start-ups focused on green fuel and grid-scale battery technologies. He also co-founded the FSHD Canada Foundation, Solve FSHD, and Project Mercury, and brings extensive knowledge in driving global progress in FSHD research.

Stuart Lai
Stuart Lai brings more than 30 years of experience in software engineering and data infrastructure from leading financial and technology firms, including Goldman Sachs, Refinitiv, and Crux Informatics. A computer and electrical engineer, he has built large-scale systems that support global analytics and data access. As Chair of the FSHD Society's Patient Access and Advocacy Committee, and a new member of the Board of the Global FSHD Innovation Hub, Lai combines his deep technical expertise with a personal commitment to improving access and outcomes for people living with FSHD.
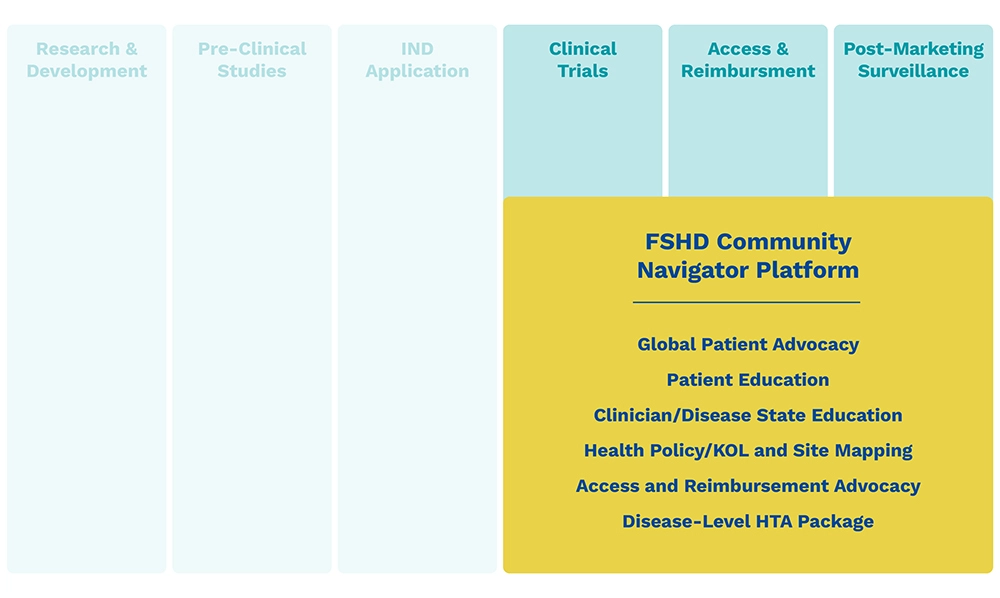
Why the Innovation Hub Was Created
The Hub serves as a comprehensive end-to-end partner for biopharma companies, providing innovative solutions along the FSHD product lifecycle that solve key challenges in FSHD:
Poor FSHD trial capacity
Multiple promising therapies for FSHD are in development but there is a lack of capacity in the FSHD research community due to:
- Lack of operational experience and excellence at sites
- Wide gap between best sites and average sites in optimized capacity and delivery on trial commitments
- Lack of landscape and feasibility understanding to identify qualified early phase trial sites with trained expertise
- Slow site contracting process resulting in trial start-up delays
- Delays in transition from early phase to pivotal trial
Many patients are unable to access approved therapies
It is expected that many patients globally will not have access. Payers in many countries will implement rigid and exclusive reimbursement criteria. Overcoming this means we must address:
- Siloed patient registries and data with lack of operational excellence and experience resulting in limited accessibility and completeness of data
- Lack of patient population data and disease underdiagnosed
- Education — disease state not well understood by clinical and patient community
- Lack of post-marketing real-world data (RWD) to support innovative risk sharing and reimbursement
Contact Us
How can we help?
Please select a topic below related to your inquiry. We look forward to speaking with you:

Support

Call Us
© 2025 FSHD Solutions